Modern quantum mechanics tells us that electrons are not localised in space, but behave rather as waves, and their classical properties such as positions, momenta, energies and angular momenta may be only fuzzily defined (i.e., we can only know probabilistically the outcomes of their measurements). The electrons only radiate light when they are stimulated to by interacting with passing light (stimulated emission), or by interacting with the vacuum (spontaneous emission).
We expect quantum physics to reduce to classical physics in some limit; the nature of this limit is not well established - it could be the limit of large objects or many interactions with the environment. Rydberg atoms are atoms with an electron excited to a high energy state, such that the electron is likely to be found far away from the nucleus of the atom. In these two recent papers, atoms are excited to a superposition of such Rydberg states so that their outer electrons are fairly localised in space, and orbit the atomic nucleii, much in the way of the Bohr atom. This is a classical limit, in the sense that the electron follows a classical trajectory, however, spontaneous emission of light from such atoms is fairly well suppressed (since the rate of emission scales in inverse proportion of the energy cubed). It actually seems that the lifetimes of the orbits are limited by the dephasing of the wavepacket (i.e., the orbit's quantum nature becomes important), rather than by spontaneous emission.
Nevertheless, it would be interesting to look at the spontaneous emission as a function of the expected angular momentum or position, and compare it to the classical radiation from an orbiting charge. Actually, I think I may attempt these calculations myself. I would normally be loath to describe my research ideas in a public forum incase I get scooped; however, I suspect that no-one reads this blog anyway.
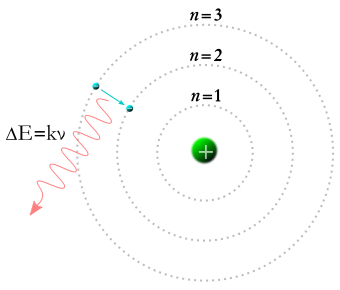
I conclude with a picture of a Bohr atom taken from Wikipedia:




I'm reading you, Andy, don't feel unloved!
ReplyDeleteWell...at least I pop by now and then...but I'm unlikely to scoop your results...
-- Margaret Harris